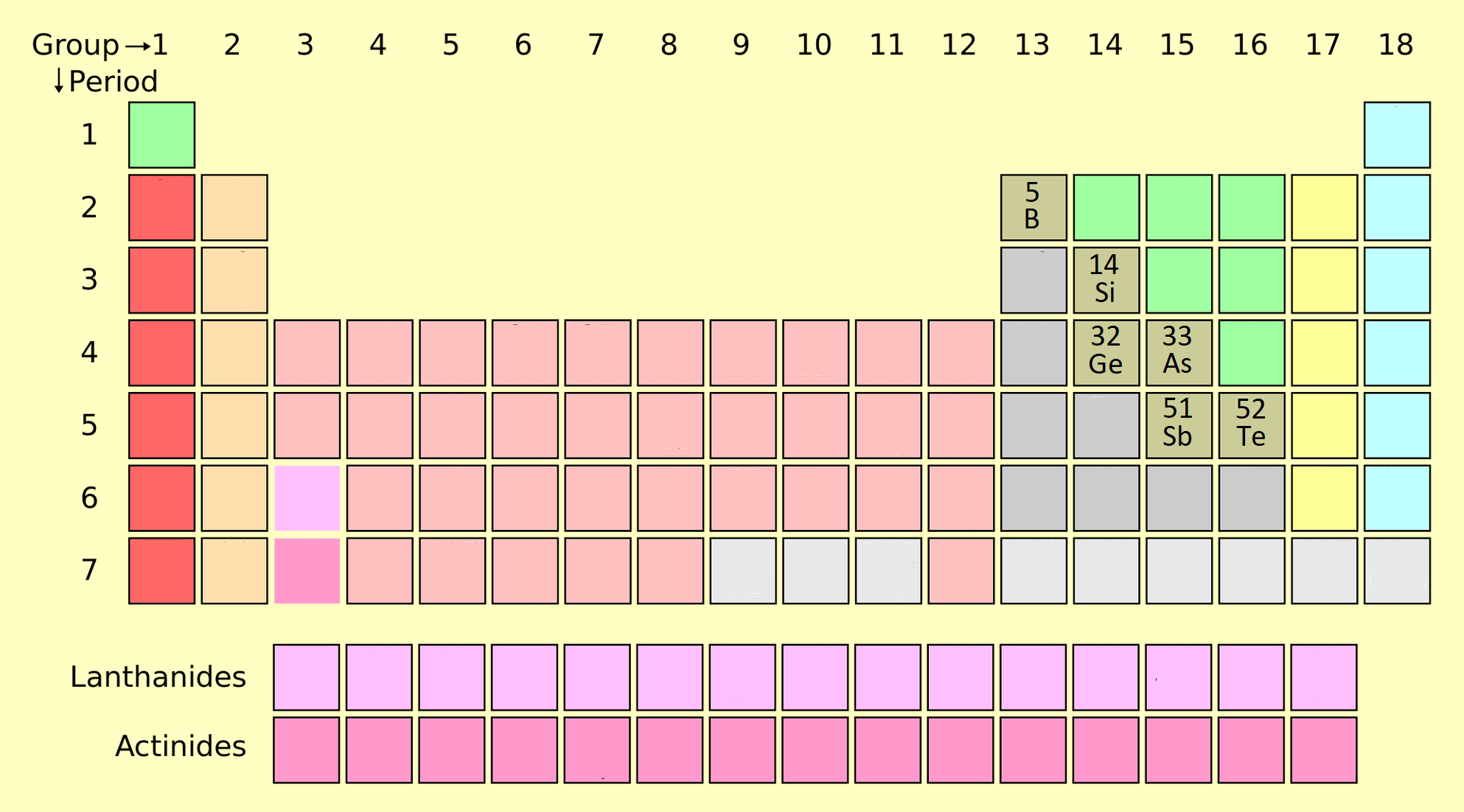
Metalloids On The Periodic Table Definition . On the periodic table, the. Because these elements have intermediate properties, it's. The metalloid group separates the metals from the nonmetals. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Elements to the left are metals and nonmetals are. Each metalloid element takes many forms, but has at least one. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The metalloids are boron, silicon, germanium, arsenic, antimony, and. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. This periodic table shows the three different groups of elements. Metalloids can also be called semimetals.
from newtondesk.com
On the periodic table, the. Elements to the left are metals and nonmetals are. Each metalloid element takes many forms, but has at least one. This periodic table shows the three different groups of elements. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Metalloids can also be called semimetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. The metalloids are boron, silicon, germanium, arsenic, antimony, and.
Metalloids (Periodic Table) Properties, Uses, & Facts
Metalloids On The Periodic Table Definition The metalloids are boron, silicon, germanium, arsenic, antimony, and. This periodic table shows the three different groups of elements. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The metalloid group separates the metals from the nonmetals. Because these elements have intermediate properties, it's. Elements to the left are metals and nonmetals are. The metalloids are boron, silicon, germanium, arsenic, antimony, and. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. On the periodic table, the. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids can also be called semimetals. Each metalloid element takes many forms, but has at least one.
From brokeasshome.com
Metalloid On The Periodic Table Metalloids On The Periodic Table Definition Metalloids can also be called semimetals. Each metalloid element takes many forms, but has at least one. This periodic table shows the three different groups of elements. The metalloid group separates the metals from the nonmetals. Because these elements have intermediate properties, it's. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals.. Metalloids On The Periodic Table Definition.
From byjus.com
How many metals, metalloids and nonmetals are there in the third period Metalloids On The Periodic Table Definition Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. On the periodic table, the. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Each metalloid element takes many forms, but has at least one. The metalloid group separates the metals from. Metalloids On The Periodic Table Definition.
From pediabay.com
Periodic Table Metals, Nonmetals & Metalloids Pediabay Metalloids On The Periodic Table Definition This periodic table shows the three different groups of elements. The metalloids are boron, silicon, germanium, arsenic, antimony, and. The metalloid group separates the metals from the nonmetals. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having. Metalloids On The Periodic Table Definition.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts Metalloids On The Periodic Table Definition Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The metalloid group separates the metals from the nonmetals. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table.. Metalloids On The Periodic Table Definition.
From www.engineeringchoice.com
Where Are Metalloids on The Periodic Table? Metalloids On The Periodic Table Definition On the periodic table, the. Because these elements have intermediate properties, it's. This periodic table shows the three different groups of elements. The metalloid group separates the metals from the nonmetals. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. The metalloids or semimetals are elements with properties intermediate between the. Metalloids On The Periodic Table Definition.
From utedzz.blogspot.com
Periodic Table Nonmetals Periodic Table Timeline Metalloids On The Periodic Table Definition Because these elements have intermediate properties, it's. This periodic table shows the three different groups of elements. The metalloid group separates the metals from the nonmetals. Each metalloid element takes many forms, but has at least one. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. On the periodic table, the. Metalloids can also be. Metalloids On The Periodic Table Definition.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts Metalloids On The Periodic Table Definition Metalloids can also be called semimetals. This periodic table shows the three different groups of elements. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. Elements to the left are metals and nonmetals are. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic. Metalloids On The Periodic Table Definition.
From www.xometry.com
Metalloids Properties and Uses Xometry Metalloids On The Periodic Table Definition Metalloids can also be called semimetals. This periodic table shows the three different groups of elements. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Each metalloid element takes many forms, but has at least one. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. A. Metalloids On The Periodic Table Definition.
From www.meadmetals.com
What’s the Difference Between Metals, Nonmetals, and Metalloids? Metalloids On The Periodic Table Definition Elements to the left are metals and nonmetals are. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Each metalloid element takes many forms, but has at least one. Metalloids can also be called semimetals. The metalloid group separates the metals from the nonmetals. A metalloid is an element that has. Metalloids On The Periodic Table Definition.
From www.periodictableprintable.com
Metals Nonmetals And Metalloids Located On Periodic Table 2024 Metalloids On The Periodic Table Definition Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloid group separates the metals from the nonmetals. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals.. Metalloids On The Periodic Table Definition.
From www.thoughtco.com
Semimetals or Metalloids List of Elements Metalloids On The Periodic Table Definition Elements to the left are metals and nonmetals are. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Each metalloid element takes many forms, but has at least one. The metalloids are boron, silicon,. Metalloids On The Periodic Table Definition.
From jawapanwed.blogspot.com
Metalloids Definition Physical Science jawapan wed Metalloids On The Periodic Table Definition Each metalloid element takes many forms, but has at least one. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloids can also be called semimetals. The metalloids or semimetals are elements with properties. Metalloids On The Periodic Table Definition.
From brokeasshome.com
Metalloids Located On The Periodic Table Metalloids On The Periodic Table Definition Metalloids can also be called semimetals. On the periodic table, the. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Because these elements have intermediate properties, it's. This periodic table shows the three different groups of elements.. Metalloids On The Periodic Table Definition.
From utedzz.blogspot.com
Periodic Table Metalloids Examples Periodic Table Timeline Metalloids On The Periodic Table Definition The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. Elements to the left are metals and nonmetals are. Each metalloid element takes many forms, but has at least one. The metalloids are boron, silicon, germanium, arsenic, antimony,. Metalloids On The Periodic Table Definition.
From www.britannica.com
alkali metal Definition, Properties, & Facts Britannica Metalloids On The Periodic Table Definition The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. The metalloids are boron, silicon, germanium, arsenic, antimony, and. This periodic table shows the three different groups of elements. The metalloid group separates the metals from the nonmetals. Each metalloid element takes many forms, but has at least one. Because these elements have intermediate properties, it's.. Metalloids On The Periodic Table Definition.
From periodictable.me
Labeled Periodic Table of Elements with Name Dynamic Periodic Table Metalloids On The Periodic Table Definition Because these elements have intermediate properties, it's. Elements to the left are metals and nonmetals are. On the periodic table, the. The metalloid group separates the metals from the nonmetals. The metalloids are boron, silicon, germanium, arsenic, antimony, and. This periodic table shows the three different groups of elements. A metalloid is an element that has properties that are intermediate. Metalloids On The Periodic Table Definition.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Metalloids On The Periodic Table Definition The metalloid group separates the metals from the nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids can also be called semimetals. Each metalloid element takes many forms, but has at least one. The metalloids or semimetals are located along the line between the metals and nonmetals in the. Metalloids On The Periodic Table Definition.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Metalloids On The Periodic Table Definition The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. On the periodic table, the. Metalloids can also be called semimetals. This periodic table shows the three different groups of elements. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Each metalloid element takes many forms,. Metalloids On The Periodic Table Definition.